The compressibility factor (Z), also known as the compression factor, is the ratio of the molar volume of a gas to the molar volume of an ideal gas at the same temperature and pressure. It is a useful thermodynamic property for modifying the ideal gas law to account for the real gas behavior.
The compressibility factor is defined as:
-

where 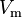 is the molar volume,
is the molar volume, 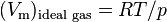 is the molar volume of the corresponding ideal gas,
is the molar volume of the corresponding ideal gas, 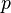 is the pressure,
is the pressure,  is the temperature, and
is the temperature, and 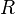 is the gas constant.
is the gas constant.